When a volume of air is compressed its temperature A. The answer is anyone who can solve the equation.

Question Video Calculating The Work Done To Compress An Ideal Gas Nagwa
C 100 degrees C.

. P is pressure V is volume n is the amount of gas mass number of molecules etc R is a constant and T is the temperature in Kelvins. I thought if any volume of air leaks to the surrounding its volume would increase by 207 times ie. An equal volume of air that is twice as hot has a temperature of A 0 degrees C.
D 273 degrees C. When air is compressed rapidly the temperature increases according to the laws of thermodynamics. Gases and Compressed Air - Air LNG LPG and other common gas properties pipeline capacities sizing of relief valves.
Compression of air is caused under increased pressure which leads to rise a proportional increase in heat. Decreases C neither increases nor decreases. 2 m3 108 216 m3.
The universal gas law is PV nRT. 2 m3 of air is heated from 22oC to 43oC. The combination law explains what happens to air when its compressed into a smaller volume.
30 When a volume of air is compressed its temperature A increases B decreases C. Am I doing this right. 30 when a volume of air is compressed its temperature.
Balloons shrink when placed inside a beaker of cold liquid nitrogen. E None of the above choices are correct. The increase in heat leads to a rise in temperature of the system.
Or simply VT constant When the gas is compressed it means that the volume decreases. The volume correction factor is 108 and the new volume can be calculated as. If a solid object radiates more energy than it absorbs its A.
The volume of a gas rises as its temperature is raised. Compression of air is caused under increased pressure which leads to rise a proportional increase in heat. Pages 6 This preview shows page 4 - 6 out of 6 pages.
According to Charles - GayLussacs Law the volume of a fixed amount of gas maintained at constant pressure is directly proportional to its temperature. Temperature compressed air Upvote12Downvote2ShareAnswer itWhen you compress it the molecules are forced come closer each other that makes decrease the volume air occupies. If the volume is compressed isothermally that is by allowing it to equilibrate with its surroundings then the temperature is constant no change.
When a volume of air is compressed its temperature Posted on March 30 2021 by admin Frequently asked questions. So since the pressure and the amount of gas is constant Volume is proportional to temperature. 207times V_1 V_2 because the tank can contain 207 times more air than the atmosphere which has roughly 1 bar of pressure.
However the resulting temperature ends up being 40 degrees Celsius again. When air is compressed rapidly its volume decreases leading to increase in temperature. Course Title ME 6502.
Internal energy decreases D. As Charles - GayLussacs Law states we could predict that the temperature of the. Then as shown here T 2 T 1 P 2 P 1 γ 1 γ This assumes you dont leak heat to the walls probably not such a good assumption.
Answer 1 of 3. A volume of air has a temperature of 0 degrees Celsius. The pressure can then be calculated using the ideal gas equation or.
Likewise people ask when air is compressed what happens to. If you assume adiabatic compression the law is P V γ k where γ C P C V is the ratio of specific heats and is usually about 14 for air. Pressure multiplied by volume divided by temperature equals a constant.
Internal energy and temperature decrease B. None of the above choices are true. B 64 degrees C.
The temperature decreases. In a limited sense yes. Poor absorber of radiation.
Normally when compressing a gas the temperature increases. When air is compressed rapidly the temperature increases according to the laws of thermodynamics. A sealed syringe contains 10 10-6 m 3 of air at 1 10 5 Pa.
It tells us that when air is compressed the pressure and temperature of the air increases as the volume of the space containing air decreases. The compression isothermal constant temperature only volume decreases. When air is compressed rapidly its volume decreases leading to increase in temperature.
PVT k.

The Top 10 Faqs About Compressed Air Fun Facts About Compressed Air

What Is Compressed Air Atlas Copco Usa
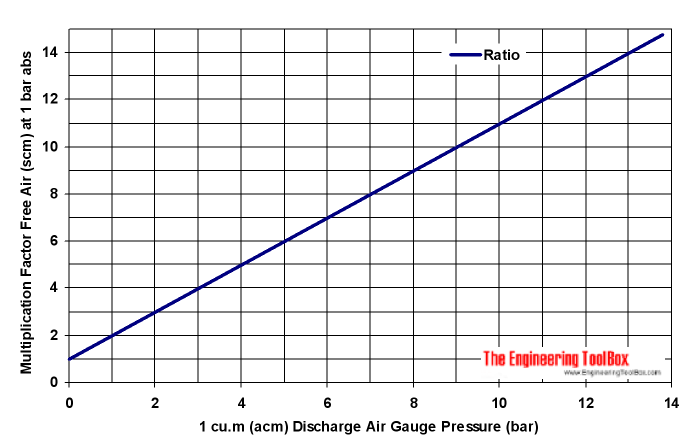
Compressed Air Vs Free Air Compression Ratio
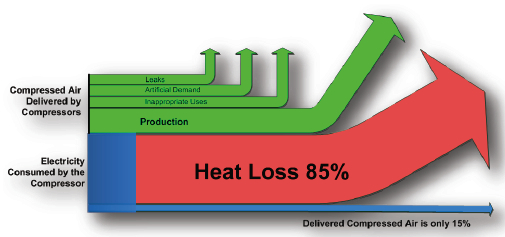
Heat Recovery And Compressed Air Systems Compressed Air Best Practices
0 Comments